Lactose-free milk: production technologies

- | آتاماد |
- Viewer: 253
Technologies used for LF milk production include filtration (membrane separation, chromatography, and crystallization), and lactose hydrolyzing (batch and aseptic).
• Separation techniques
1) Membrane separation
Ultrafiltration (UF) and nanofiltration (NF) processing is mainly applied to separate lactose from milk. Non-thermal technology, Membrane filtration effectively removes compounds like lactose and drug residues in milk that may interfere with the product’s quality, texture, and shelf-life. The membrane separation process results in a 40% reduction of lactose by separation. The filtration process does not require specialized knowledge to be operated.
2) Chromatography
Chromatography may be used to separate components like lactose based on differences in the flow velocities of different components of a liquid. This method has been developed to specifically separate lactose from milk and whey by strong cation exchange resins. Employing chromatography technology is more difficult for dairy plants to adopt.
3) Crystallization
By using the crystallization process, the lactose is concentrated to a high total solid content so that the lactose becomes saturated and crystallizes, the lactose crystals are subsequently separated using centrifugation. Using the crystallization method to produce LFM is not likely due to the high viscosity of concentrated milk. So, the method is mainly applied in commercial lactose production.
• Hydrolysis
Today, two methods, batch and aseptic, are applied to produce lactose-free milk, which is related to milk sterilization before and after hydrolyzation respectively. In both methods, soluble lactase enzyme is used. Increasing sweetness is one of the side effects of glucose and galactose production due to lactose hydrolyzation.
The lactases used for commercial production of LFM are available from a number of sources like microbial- or fungal origin.
β-galactosidase
Using microbial lactases for hydrolyzing lactose in dairy products was first reported in 1950. Two types of commercial lactases are available, neutral lactase with fungal origin is derived from the dairy yeast Kluyveromyces lactis and its close relatives Saccharomyces lactis, K. marxianus, and K. fragilis, performing best under low to ambient temperatures and neutral pH conditions. And other commercial lactases, acid lactase, with bacterial (Bacillus circulans) and fungal origin (Aspergillus oryzae), are less suitable for the hydrolysis of lactose because of the different pH or temperature optimums, thus, they are mainly sold either as nutritional enzymes or for the production of galactooligosaccharides (GOS).
Recently, a recombinantly new type of lactase from Bifidobacterium bifidum produced in Bacillus licheniformis entered the market and is claimed to have a broader pH tolerance. In recent years, galactosidase produced by genetic modification has also entered the market, like the shortened version of Bifidobacterium lactase, which is cloned with the Bacillus host strain, or its cloned version, which is produced from k.lactis, but those have more specific activity.
Besides the organisms mentioned before, LAB are promising lactase sources due to their generally recognized-as-safe (GRAS) status. Interestingly, the natural microflora of yogurt is composed of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus (S. thermophilus) are sources of lactase activity. As a result, lactose from yogurt is digested better by LI people than lactose from other sources such as milk. LAB lactases, especially, the lactase from S. thermophilus is highly active, heat-tolerant, and stable during long-term storage, having excellent properties when compared to yeast enzymes. However, its expression and activity are strongly dependent on the environment and culturing conditions. Interestingly, the activity of lactase from S. thermophilus was 33% less in milk than under optimal conditions due to an unfavorable ionic environment in milk. Furthermore, compared to other lactases, S. thermophilus’ lactase is quite sensitive to low pH. Here, optimization of the enzyme’s properties, like, using RMS (random mutagenesis and screening), could result in useful strains for the production of LF yogurt. Acid-tolerant lactase expressed by starter culture could facilitate the production of LF yogurt with no need for additional lactase. A comparison of over 60 lactases showed that Lactococcus lactis (L. lactis, formerly Streptococcus lactis or Streptococcus cremoris), Lactobacillus delbrueckii subsp. bulgaricus and Leuconostoc citrovorum are also excellent sources of lactase enzymes.
Cold-adapted lactases or enzymes from extremophiles have gained attention because the hydrolysis of lactose using the batch process is typically carried out at 4–8°C. Here, cold-active enzymes could dramatically reduce the required reaction time compared to yeast enzymes.
Besides the general advantages of thermostable enzymes, the risk of microbial contamination is reduced at high temperatures through lactose hydrolysis. Thus, in the batch process, thermostable lactases could be incubated before pasteurization, avoiding a long incubation time at 4–8°C.
All in all, both cold-active and thermostable lactases have excellent properties to find application in the dairy industry. The recombinant expression of extremophile enzymes in GRAS organisms is a potential solution for allowing them to be commercialized. The availability of extremophilic lactases could reduce costs and environmental impact by saving production time and resources. Nevertheless, products claiming to be completely free of genetic modification (GM-free) are gaining increasing popularity among people.
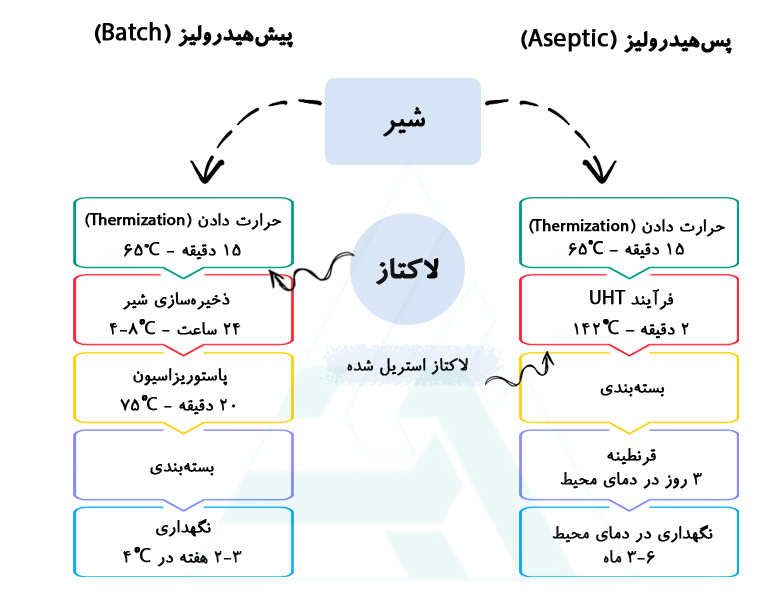
1. Batch or pre-hydrolysis method
In this process, neutral lactase is added to raw milk and incubated for about 24h under slow stirring to prevent creaming. this process has to be performed at cooled conditions of 4–8°C to avoid microbial growth. Sections like homogenization and standardization are usually done before the heat treatment. The advantage of this method is that the enzyme becomes inactive during pasteurization and sterilization, and as a result, enzyme activity is not observed in the final product.
Important aspects to consider when using the batch process for making LF milk:
• To reach the required boundary for LF, the enzyme dosage should be adequate due to the low temperature of the incubation and limited time. Therefore, the enzyme dosage is relatively high. Enzymes available for the batch process are selected for their quite high activity at low temperatures and neutral pH. The process control is high since the enzyme dosage or incubation time may be adapted during the process.
• The occupation of a tank in the plant is required for batch incubation. Therefore, the process is discontinuous, which may give rise to a problem for some factories. A lactase with a higher specific activity under these conditions may help shorten the production time and, hence, may increase the throughput of the factory. To prevent microbial spoilage, high-quality milk should be used since the pasteurization is postponed for a day.
• The limited time of storage at refrigerated conditions and the pasteurization/sterilization after the enzyme incubation leads to the inactivation of most enzymatic activities. Therefore, the product produced with this process is quite insensitive for possible side activities in the enzyme preparation.
• To generate an exact sweetness, a part of the lactose removes by chromatography or (ultra and nano) filtration techniques combined with the hydrolysis of the remaining lactose.
2. Aseptic or post-hydrolysis method:
In the post-hydrolysis process, the sterilization of milk is done by the UHT procedure, and then just before packaging, sterile lactase is added to the milk, so the lactose hydrolyzation will take place in the packaging. UHT milk is often kept in quarantine for about 3 days at ambient temperature, so there is enough time for complete hydrolysis before the milk is shipped to the retailer. Since there is no quarantine period for pasteurized milk, the aseptic process is not used for this type of LF milk. To obtain sterile lactase, there are two different procedures; in the first procedure, the manufacturer of the enzyme pre-sterilizes the lactase, and for the sterile injection special sterile dosing equipment is required. In the other procedure, the enzyme is filter-sterilized just before adding to the sterile milk.
Important aspects to consider when using the aseptic process for making LF milk:
• The dosage of the enzyme can be much lower compared to the batch process since both the incubation time and temperature are higher. Since the enzyme is only active in the final milk package, process control is absent.
• This process requires consumable costs and special equipment, and also highly skilled operators for the in-factory filtration, s to prevent microbial contamination during lactase injection. However, the process can be operated full-continuous when organized properly, and that is a major advantage for factories that require high throughput.
• The aseptic process for making LF UHT milk could only be fully developed after major improvements in the quality of the lactase enzymes. In addition to the removal of proteolytic side activity, it was also found that arylsulfatase side activity may lead to medicinal off-flavors during storage due to p-cresol formation. A producer of LF UHT milk should consider using only the highest quality lactases to prevent such problems.
• An increased presence of monosaccharides happens as a result of lactose hydrolysis, thus the Maillard reaction is more efficient. This results in off-flavors, reduced shelf life, and nutritional value of LF milk when compared to regular milk, especially when stored at increased temperatures. Recent data show that the storage temperature and choice of the lactase are much more relevant for determining shelf life than the production process (batch or aseptic). Therefore, milk browning during storage is largely independent of the production process that is used.
References:
- Peter J. T. Dekker, Damiet Koenders, and Maaike J. Bruins. “Lactose-Free Dairy Products: Market Developments, Production, Nutrition and Health Benefits.” Nutrients, 2019 Mar; 11(3): 551; doi:10.3390/nu11030551.
- Lactose-free milk: Lactose intolerance, nutrition and process technology, by Signe Christerson, 2021.
GET IN TOUCH
Copyright © 2023 Atamad.com All right reserved
Website design and SEO services by Seohama team – Web hosting by Sarverhama
Copyright © 2023 Atamad.com All right reserved
Website design and SEO services by Seohama team – Web hosting by Sarverhama